Background: Blinatumomab (blina) improves outcomes in R/R CD19+ ALL compared to chemotherapy. However, the overall response rate to blina was 44%, and median overall survival (OS) is just 7.7 mos (Kantarjian. NEJM. 2017). Preclinical studies show increased PD-L1 expression on leukemic blasts potentially contributes to relapse after blina. The addition of PD-1 +/- CTLA-4 blockade to blina leads to increased in vitro T cell proliferation and enhanced cytotoxicity (Feucht. Oncotarget 2016). Thus, blockade of co-inhibitory pathways may enhance blina efficacy in vivo. We describe results of a multi-center phase I study combining blina with immune checkpoint inhibitors (ICIs) targeting PD-1 (nivolumab) +/- CTLA-4 (ipilimumab).
Methods: In a dose-escalation study, we evaluated the safety, tolerability, and preliminary efficacy of blina with nivolumab (nivo) +/- ipilimumab (ipi) using a 3+3 design. Pts ³16 years-old with R/R CD19+ B-ALL or mixed phenotype acute leukemia (MPAL) were eligible. Pts ³60 years could be untreated. Table 1 presents the dose escalation schema. Expansion cohorts of 6 pts were planned at the maximum tolerated dose (MTD) for blina + nivo AND blina + nivo + ipi. Pts received up to 5 cycles of blina and 1 year of ICIs. Pts removed from the study during the blina lead-in (days 1-10) were replaced. Dose-limiting toxicities (DLTs) were defined as grade 3+ non-hematologic toxicities requiring the permanent discontinuation of treatment in the first 42 days. Primary endpoints were toxicities and MTD. Secondary endpoints included complete remission (CR), MRD-negativity at a sensitivity of 0.01%, duration of response and OS from treatment initiation.
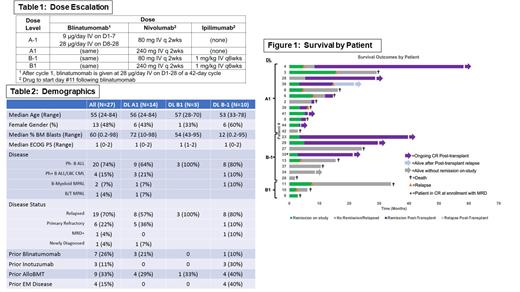
Results: Twenty-seven pts were enrolled to 3 dose levels (14 DLA1, 3 DLB1, and 10 DLB-1) from September 2017-December 2022. Six pts were replaced (4 prior to ICI dosing and 2 requiring subsequent therapy in the DLT window). The median age of enrolled pts was 55 (range 24-84), 14 were male (52%), and median baseline BM blasts were 60% (range 0.2-98%). Baseline characteristics are presented in Table 2. A single DLT (G4 infusion reaction and G3 hypotension) occurred among 6 pts at DLA1. Based on this safety and efficacy data, blina + nivo was expanded to include 6 additional pts without additional DLTs. There were 2 DLTs at DLB1 (G5 pneumonitis and G2 GVHD), which led to de-escalation to DLB-1, where there was 1 DLT (G3 delirium). Grade 2+ immune-related adverse events attributable to ICIs included pneumonitis (G2+G5), rash (G2+G3), transaminitis (G2+G3), colitis (G3), and hypothyroidism (G2). Grade 3+ adverse events attributable to blina included neutropenia (5-G4+3-G3), dysphasia (2-G3), weakness (G3), seizure (G4), and transaminitis (1-G4+4-G3). Among 22 pts evaluable for response, the CR rate was 68% (100% MRD-negative). Among 13 pts with >50% BM blasts at baseline, 62% achieved CR. Pts with B-myeloid MPAL (0/2) and a history of extramedullary disease (1/4) were less likely to respond. Nine responders subsequently relapsed including 3 with isolated extramedullary disease and 2 with CD19-negative disease. As shown in Figure 1, relapse-free survival (RFS) at 1 year was 27% (95% CI 10-46), while OS at 1 year was 63% (95% CI 38-79). Twelve pts underwent allogeneic blood or marrow transplant (alloBMT) following study treatment. At 1 year for alloBMT pts, RFS was 51% (95% CI 19-76) and OS was 61% (95% CI 26-83). Ongoing analyses of changes in T cell subpopulations, co-signaling molecule expression, and single cell RNA seq results will be presented.
Conclusions: Combination therapy with blina and ICIs in R/R ALL is safe, feasible, and associated with a high MRD-negative response rate. Long-term survival was promising in comparison to prior results with blina monotherapy, especially following consolidation with alloBMT. A randomized trial of blina +/- nivolumab is needed to confirm the benefit of this combination.
OffLabel Disclosure:
Webster:Servier: Consultancy; Pfizer: Consultancy. Luskin:Novartis: Honoraria; Novartis: Research Funding; Pfizer: Honoraria; Jazz: Honoraria; AbbVie: Research Funding. Rimando:Merck: Current equity holder in publicly-traded company. Zeidan:Notable: Consultancy, Honoraria; Orum: Consultancy, Honoraria; Daiichi Sankyo: Consultancy, Honoraria; Mendus: Consultancy, Honoraria; Otsuka: Consultancy, Honoraria; Seattle Genetics: Consultancy, Honoraria; Servier: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; AbbVie: Consultancy, Honoraria; Astellas: Consultancy, Honoraria; Boehringer-Ingelheim: Consultancy, Honoraria; Jazz: Consultancy, Honoraria; Takeda: Consultancy, Honoraria; BeyondSpring: Consultancy, Honoraria; Celgene/BMS: Consultancy, Honoraria; Pfizer: Consultancy, Honoraria; Zentalis: Consultancy, Honoraria; Taiho: Consultancy, Honoraria; Geron: Consultancy, Honoraria; BioCryst: Consultancy, Honoraria; ALX Oncology: Consultancy, Honoraria; Chiesi: Consultancy, Honoraria; Kura: Consultancy, Honoraria; Gilead: Consultancy, Honoraria; Syndax: Consultancy, Honoraria; Schrödinger: Consultancy, Honoraria; Regeneron: Consultancy, Honoraria; Lox Oncology: Consultancy, Honoraria; Syros: Consultancy, Honoraria; Tyme: Consultancy, Honoraria; Astex: Research Funding; Shattuck Labs: Research Funding; Foran: Consultancy, Research Funding; Ionis: Consultancy, Honoraria; Amgen: Consultancy, Honoraria; Janssen: Consultancy, Honoraria; Genentech: Consultancy, Honoraria; Epizyme: Consultancy, Honoraria; Incyte: Consultancy, Honoraria; Agios: Consultancy, Honoraria. DeAngelo:Gilead: Honoraria; Incyte: Honoraria; Pfizer: Honoraria; Takeda: Honoraria; AbbVie: Research Funding; Servier: Honoraria; Jazz: Honoraria; Kite: Honoraria; Autolus: Honoraria; Novartis: Research Funding; Novartis: Honoraria; Amgen: Honoraria; Blueprint: Honoraria; GlycoMimetics: Research Funding; Blueprint: Research Funding. Luznik:Talaris Therapeutics: Consultancy; Precision Biosciences: Consultancy; WindMiL therpeutics: Patents & Royalties; Genentech: Research Funding; Gilead Sciences: Consultancy; Rubius Therapeutics: Consultancy. Gojo:Gilead: Research Funding; Scimentum: Consultancy, Membership on an entity's Board of Directors or advisory committees; Merck: Research Funding; Incyte: Research Funding; Clearview: Consultancy, Membership on an entity's Board of Directors or advisory committees; Amgen: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; MJH Healthcare Holdings: Consultancy, Membership on an entity's Board of Directors or advisory committees; Nkarta: Consultancy, Membership on an entity's Board of Directors or advisory committees.
Immune checkpoint inhibitors are off-label for R/R B ALL and the subject of this trial

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal